Current Location:HomeCMC ProcessBiologics Process Development
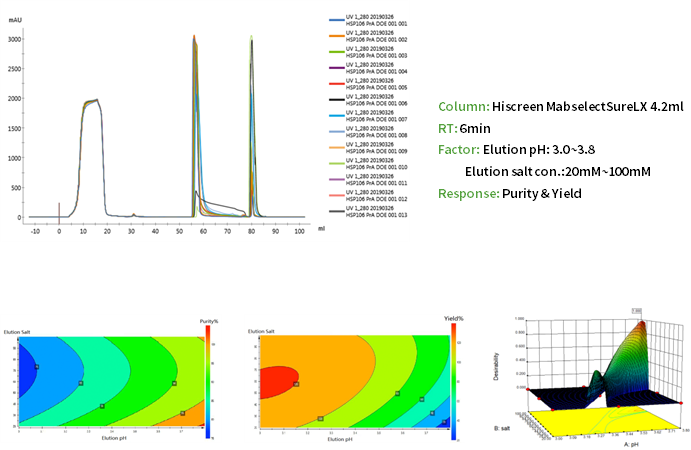
Downstream Process Development Platform has wide experience in purification process development, scale-up and manufacturing for antibodies and recombinant proteins. The whole process follows the ICH Guidelines and QbD Concept, and normally achieves a highly efficient, robust and scalable downstream process in 8-12 weeks, with an overall yield of around 70%.
Equipped with intelligent protein chromatography systems such as AKTA Pure, Avant , the platform has rich experience in process development of biologics like monoclonal antibodies, bispecific antibodies, multiple antibodies, fusion proteins, ADCs.

Email:healsunbd@hs-biopharm.com


Copyright © 2023 杭州皓陽生物(wù)技(jì )術有限公司All Rights Reserved
京ICP證000000号
 浙公網安(ān)備 33011002011493号
技(jì )術支持:杭州網站制作(zuò)
浙公網安(ān)備 33011002011493号
技(jì )術支持:杭州網站制作(zuò)