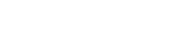Current Location:HomeGMP Pilot ProductionBiologics
Healsun Biopharm has 5,000㎡ of GMP production line of DS (Drug Substance) and DP (Drug Product ) in compliance with FDA, EMA, NMPA, and cGMP requirements, providing service of toxicology batch samples for biologics, GMP samples for IND filing and clinical trials.
The annual production capacity for DS can reach up to 20 batches, with each batch being 3,000 grams. For DP, the annual production capacity can reach up to 40 batches, with each batch consisting of 10,000 units.
We can deliver from antibody DNA to toxicology samples within 7 months and fulfill Pre-IND preparation within 12 months.


Email:healsunbd@hs-biopharm.com


Copyright © 2023 杭州皓陽生物(wù)技(jì )術有限公司All Rights Reserved
京ICP證000000号
 浙公網安(ān)備 33011002011493号
技(jì )術支持:杭州網站制作(zuò)
浙公網安(ān)備 33011002011493号
技(jì )術支持:杭州網站制作(zuò)